Understanding the ISO 13485 Standard
Medical devices are any instruments, machines, or implants that help diagnose, treat, or prevent medical conditions. When it comes to medical device manufacturing, safety and quality are non-negotiable and regulatory requirements are increasingly stringent. Every step of the product life cycle must pass internationally agreed-upon standards to ensure it’s safe to use in medical procedures and emergencies. To meet these standards, organizations are required to demonstrate their quality management systems; many of them do so by using ISO 13485.
What Is ISO 13485?
ISO is a global organization that sets specific quality management standards for various industries, including agriculture, medical, and health care. The latest version of its regulatory standards is ISO 13485, which ensures that every medical device is made properly and without flaw. These items fall into three different categories:
- Class I: These devices pose little risk to the patient and are not meant to be life-saving. Masks, hand-held surgical instruments, elastic bandages, and surgical gloves are examples of Class I devices.
- Class II: These devices come with a higher level of assurance that they will perform the way they should. Regulated by the FDA, some examples of these include pregnancy tests, acupuncture needles, powered wheelchairs, and infusion pumps.
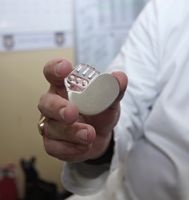 Class III: Under this classification, these devices are often life-saving measures and carry a high risk of illness or injury if not used or managed correctly. Some examples include pacemakers, pulse generators, breast implants, and external defibrillators.
Class III: Under this classification, these devices are often life-saving measures and carry a high risk of illness or injury if not used or managed correctly. Some examples include pacemakers, pulse generators, breast implants, and external defibrillators.
Why Do Organizations Need to be Certified?
When organizations get certified under the ISO 13485 standard, they acknowledge that all of their medical device manufacturing processes, designs, materials, and packages meet the parameters set by this quality management system.
Certifications are not only given to manufacturers, but also to subcontractors, suppliers, and consulting firms within the medical industry. Furthermore, the ISO 13485 standard requires that all medical personnel identify their position within the process, such as manufacturer, representative, or importer. This is not meant to bottleneck the manufacturing of medical devices, but rather streamline the process and procedures required to create safe and effective devices.
Pacific Integrated Manufacturing puts quality first. Located in Bonita, CA, this medical device manufacturing company has been supplying the industry with quality products that meet ISO 13485 standards. No matter your needs, their team aims at providing the highest level of customer service to develop convenient and cost-effective solutions. To get your free quote, call (619) 921-3464. Visit the website to learn more about their company.
About the Business
Have a question? Ask the experts!
Send your question

