COVID-19 vaccine clinical trial seeking participants in Savannah
Default Mono Sans Mono Serif Sans Serif Comic Fancy Small Caps
Default X-Small Small Medium Large X-Large XX-Large
Default Outline Dark Outline Light Outline Dark Bold Outline Light Bold Shadow Dark Shadow Light Shadow Dark Bold Shadow Light Bold
Default Black Silver Gray White Maroon Red Purple Fuchsia Green Lime Olive Yellow Navy Blue Teal Aqua OrangeDefault 100% 75% 50% 25% 0%
Default Black Silver Gray White Maroon Red Purple Fuchsia Green Lime Olive Yellow Navy Blue Teal Aqua OrangeDefault 100% 75% 50% 25% 0%
COVID-19 vaccine clinical trial seeking participants in Savannah
By Blair Caldwell | July 21, 2020 at 11:17 PM EDT - Updated July 22 at 6:47 PM
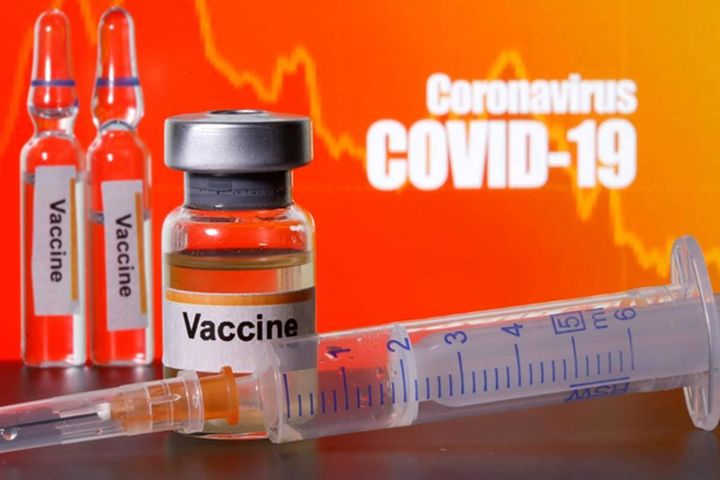
SAVANNAH, Ga. (WTOC) - A local doctor is working alongside Meridian Clinical Research to study a promising COVID-19 vaccine. But before it can go to the market, it needs further research which requires volunteers willing to help.
Richard Yaun from Richmond Hill is one of more than 60 people currently enrolled in Meridian Clinical Research’s COVID-19 vaccine study. He participated in a flu trail years ago and got the call to help again and knew his age could help their research.
“I know I don’t look 71, but both my parents lived to be 90. If I am going to be that, live to be that age or more, which I want to, then this pandemic is an obstacle that I have to get around and so does all of my family and I feel good about it,” Yaun said.
Yaun has six kids, one a teacher and another an infectious disease doctor. He knows how deeply COVID-19 has altered lives and wanted to be a part of the solution. Meridian Research Group is looking for more like him to help in phase three of the trial.
“We really need as many people as possible to participate otherwise we will be wearing these masks for the rest of our life and socially distancing for the rest of our life,” said Dr. Paul Bradley with Meridian Clinical Research.
They are looking for more than a thousand people to sign up. You must be 18 and older and will be compensated with $1,500 at a minimum. Participants will get the vaccine or a placebo, 8-10 exams, have safety calls and be asked to keep a dairy.
Dr. Bradley has been working on Phase II for months.
“This is not a live virus that we’re giving you it is simply a picture/postcard a part of the virus. You can’t watch the virus from it. It is a clinical trial, there is unknown, so far, this is not, this is Phase III already. It’s not the beginning, and again, we’ve had no serious adverse events either in Phase I or Phase II,” Dr. Bradley said.
While Yaun doesn’t know which dose he’s gotten, he says he’s felt great and appreciates the regular check-ups, tests and more. He encourages others to see if they are eligible to sign up.
“It’s a safe trail and I feel like I’m a part of the solution you know when I leave here I feel very good every time I leave here,” Yaun said.
Default Mono Sans Mono Serif Sans Serif Comic Fancy Small Caps
Default X-Small Small Medium Large X-Large XX-Large
Default Outline Dark Outline Light Outline Dark Bold Outline Light Bold Shadow Dark Shadow Light Shadow Dark Bold Shadow Light Bold
Default Black Silver Gray White Maroon Red Purple Fuchsia Green Lime Olive Yellow Navy Blue Teal Aqua OrangeDefault 100% 75% 50% 25% 0%
Default Black Silver Gray White Maroon Red Purple Fuchsia Green Lime Olive Yellow Navy Blue Teal Aqua OrangeDefault 100% 75% 50% 25% 0%
COVID-19 vaccine clinical trial seeking participants in Savannah
Dr. Bradley has been doing clinical research for three decades. Alongside Meridian Clinical Research, he’s participated in hundreds of trails and studies. He says this trial is happening fast but has shown effective.
It’s now well into Phase 2 with 600 people at ten locations; dozens of those at Dr. Bradley’s office.
“Phase 1 had a really good response. There was no serious adverse events in the subjects and as it was described, a robust response meaning that the patients who had the vaccine actually had antibody response equal to or greater than those patients who had COVID, which is exactly what we’re hoping for,” Dr. Bradley said.
While there are several vaccines being tested, Moderna’s vaccine tested by Meridian is a two-shot series that uses spike protein from the virus to prevent infection.
“Vaccines get you ready for the invader. They send you a picture postcard to know what’s going to happen and to be on the lookout so when the bad guy comes you already know what, oh you know that’s him let’s send our immune system out and let’s attack this with everything we’ve got,” Dr. Bradley said.
This vaccine is proving hopeful not just to Dr. Bradley, but others too.
“I am very optimistic. I think if their results continue to be so positive, it will probably be one of the first vaccines to be brought to the market,” Memorial Health Associate Chief Medical Officer Dr. Stephen Thacker said.
The studies will be conducted at 340 Eisenhower Dr #1200, Savannah, GA 31406.
You must be 18 years or older to participate. Participants will receive study-related exams, and an investigational COVID-19 vaccine or placebo at no cost.
Meridian is also seeking people who meet one of the following criteria:
- People who may be in close, regular contact with infected persons; this may include, but is not limited to essential workers, healthcare workers, first responders, manufacturing/factory workers, teachers, transit workers, etc.
- People with underlying health conditions, such as high blood pressure, diabetes, obesity, asthma, etc.
- Healthy individuals age 18+ with no known history of COVID-19 infection
- People age 65+
Those eligible to participate will receive compensation for participating and do not need health insurance to join.
About the Business
Have a question? Ask the experts!
Send your question

